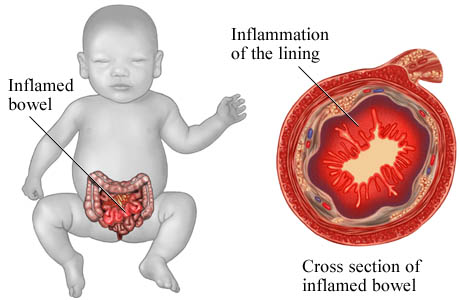
Necrotizing Enterocolitis (NEC) Symptoms & Causes
Prevention & Treatment
After the premature birth of a child, it is crucial to consider reducing the risk of developing NEC. To achieve this goal, the methods of administering hyperalimentation and oral feeds play significant roles.
Neonatologists at the University of Iowa NICU emphasized the importance of introducing small trophic oral feeds of human milk as soon as possible, even while the infant is primarily fed intravenously. This approach helps prime the immature gut to mature, preparing it to handle greater oral intake. If the mother’s milk is unavailable, human milk from a milk bank or donor can be used. In very premature infants, especially where capillary development is immature, gut mucosal cells lack sufficient nourishment from the arterial blood supply, making nutrients from the gut lumen essential.
Treatment primarily involves supportive care, including ceasing enteral feeds, gastric decompression with intermittent suction, fluid repletion to address electrolyte abnormalities and losses, blood pressure support, parenteral nutrition, and timely antibiotic therapy. Clinical monitoring is crucial, and abdominal roentgenograms should be performed every 6 hours. In cases where medical treatment alone does not halt the disease or when bowel perforation occurs, immediate emergency surgery to resect the necrotic bowel is generally necessary. Abdominal drains may be placed in very unstable infants as a temporary measure. Surgery may involve a colostomy, which could potentially be reversed at a later time. Some children may face complications like short bowel syndrome if extensive portions of the bowel are removed.
References
- ^ Sodhi C, Richardson W, Gribar S, Hackam DJ (2008). “The development of animal models for the study of necrotizing enterocolitis”. Dis Model Mech 1 (2-3): 94–8.doi:10.1242/dmm.000315. PMC 2562191. PMID 19048070.
- ^ Blueprints Pediatrics B. Marino, K. Fine
- ^ Hunter CJ, Upperman JS, Ford HR, Camerini V (February 2008). “Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC)”. Pediatric Research63 (2): 117–23. doi:10.1203/PDR.0b013e31815ed64c. PMID 18091350.
- ^ Leigh L, Stoll BJ, Rahman M, McGowan J (May 1995). “Pseudomonas aeruginosa infection in very low birth weight infants: a case-control study”. The Pediatric Infectious Disease Journal 14 (5): 367–71. doi:10.1097/00006454-199505000-00006. PMID 7638011.
- ^ Hopkins DG, Kushner JP (May 1983). “Clostridial species in the pathogenesis of necrotizing enterocolitis in patients with neutropenia”. American Journal of Hematology 14 (3): 289–95. doi:10.1002/ajh.2830140311. PMID 6846331.
- ^ a b Cotten CM, Taylor S, Stoll B, et al. (January 2009). “Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants”. Pediatrics 123 (1): 58–66. doi:10.1542/peds.2007-3423. PMC 2760222. PMID 19117861.
- ^ Schanler RJ (2001) The use of human milk for premature infants
